Life Sciences
Successful implementation of continuous flow processes 18th November 2020
By By Prof. Tom Moody (VP Technology Development and Commercialisation), Dr Megan Smyth (Senior Flow Chemist), Dr Scott Wharry (Custom and Flow Chemistry Manager), Almac Sciences and Dr Charlotte Wiles(CEO), Chemtrix BV.
Almac Sciences and Chemtrix discuss the importance of collaboration of multidisciplinary teams to successfully deliver robust continuous flow processes from lab scale to manufacture.
On paper, flow chemistry is an extremely straightforward concept- “simply pump reagents through a narrow coil or channel to react and collect the product at the outlet”. However, as any flow chemist can testify, a number of common challenges may be encountered. This article highlights some of the pitfalls and how to successfully transition an idea from paper to proof of concept and beyond to production scale.
Drivers and opportunities
Flow processes are inherently safer due to the use of lower reaction volumes, better temperature control and the ability to access higher pressures with controlled risk. The technology offers solutions to industrial priority areas including development of cleaner, less consumptive, more efficient and safer chemical processes. The advantages of continuous flow have been well documented,and are summarised in Figure 1.[1]
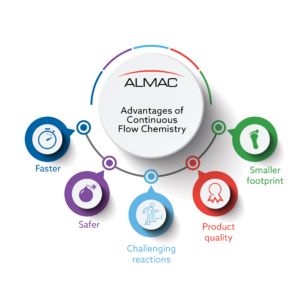
A key driver towards the adoption of flow processes for most CDMOs is maintaining a competitive edge by efficiently accessing challenging reaction classes on scales larger than are typically achievable using traditional batch processing. With the benefits of flow chemistry well recognized, one question that may arise is: “why are more processes not performed continuously?” The answer is multifaceted and includes a lack of knowledge and experience within companies, equipment availability and, importantly, the lack of an internal drive to make flow processes a reality.
Hurdles and challenges
The hurdles and challenges to implementing flow chemistry, or any new technology for that matter, can be split into technical or cultural barriers – each leading to a pitfall. With experience, these pitfalls can be used to define working practices that mitigate project risk.
Experience& Knowledge: The first challenge for any new technology is having trained and experienced staff within an organization. This means achieving competencies beyond using the new techniques and becoming experts in leveraging the associated technical and commercial benefits it can bring. Critical to change is an openness within the team to try something new in order to succeed! “But this is how we have always done it” or “we don’t do [insert chemistry] here” blocks the development of technical solutions and commercial implementation.
The success of continuous process (re-)development is reliant on a multi-disciplined project team, with chemical and chemical engineering input at an early stage to ensure informed synthetic routes are available for evaluation, together with their impact on the selection of appropriate flow architecture and unit operation integration. In addition, endorsement of the technology by the FDA[2] brings on board QA colleagues at an earlier stage than for comparative batch processes.
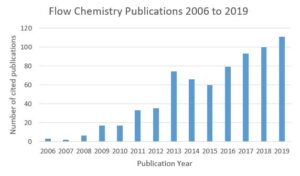
So how to get experience? The increasing volume of flow publications (Figure 2) and manufacturing process showcases is driving knowledge transfer. Partnering is also key, working together with academic and industrial partners to learn from each other and access training / hands on experience to accelerate uptake. As flow chemistry matures and is no longer limited by the number of industrially relevant examples, CDMOs are becoming faster at using in-house platforms to access wide-ranging chemistries required by their clients.
Business case & When to use flow? It can of course be a challenge to justify additional CAPEX when perfectly functional multi-purpose batch vessels are already installed. For many CDMOs, the move to implement continuous processing requires critical evaluation and demonstration of numerous advantages over existing batch capabilities.
As the pharmaceutical industry continues to develop more sophisticated and highly effective drug targets, the volume requirements of APIs (and their building blocks) have consequently decreased. This paradigm shift makes it more challenging to secure major financial investment for novel technologies.[3] Couple this with the fact that the adoption of any new technology can be hindered by the ambitious timeline deliveries set by the industry, it takes time to implement and reap the rewards of change.
The adoption of flow processing within CDMO’s is undoubtedly driven by economics, with return on investment (ROI) necessary within timelines that make the project viable. One approach is not to tie ROI to a single production campaign, but to a platform technology by developing a toolbox approach where flow is used for specific process types routinely in preference to batch, the result being modular infrastructure that can be flexible towards the production scale and chemical needs of multiple clients.
When looking at the business case more broadly, flow offers value through production cost savings (OPEX), environmental advantages such as waste reduction, reduced safety concerns and, critically, access to novel or highly challenging functional group interchanges. This brings opportunities to CDMOs likely to be limited by hydrogenation pressure and temperature extremes, cryogenic vessel size/cooling capacity and overall capacity. As a result, the use of continuous flow can add value to a CDMO’s business by unlocking access to new molecules, along with volume supply in shorter timelines.
Clogging & Fouling: It would be wrong to talk about pitfalls without covering fouling. Despite many advances in flow chemistry, one of the biggest hurdles encountered by any flow chemist at the start is reactor fouling and potential blockages. Whilst it is often thought that homogeneous solutions are a prerequisite for flow, it is important to separate this need based on the use of a static vs. dynamic flow reactor and towards the choice of feed dosing system (ie choice of pumps).
Reactor fouling can occur for several reasons: solubility of intermediates / by-products; incompatibilities with materials of construction; stagnant zones in mixing elements / connectors / regulators; and moisture ingress via the dosing system. It can also be due to impurities present in solvents, as shown recently with the reports of Poly-THF in commercially available THF resulting in reactor clogging.[4] It is essential that the root cause of potential fouling events is evaluated at lab scale so that appropriate measures can be put in place to prevent or mitigate risk on scale-up. Such measures centre on appropriate hardware selection in terms of materials of construction, flow path geometries and dosing line design, but can also include feed quality and stability assessments.
The prevention and resolution of clogging issues can be supported by appropriate analytical methods. Process Analytical Technology (PAT)[5] facilitates in-line, real-time monitoring of a process, finding particular value as a system ‘health check’ for manufacturing campaigns.
Continuous flow as a collaborative industry
As CDMOs build flow capabilities internally (or externally through partnership) there are cultural changes to overcome, as most of the required skillsets (of chemists, analysts, chemical engineers, QA staff, etc.) will be batch-centric! Alongside the mind-set change needed when performing retrosynthetic and process design, implementation of continuous flow processes relies on successful partnership with multi-disciplinary teams. Building a team of chemists and chemical engineers, who work closely together from the lab development stage right through to scale-up mitigates the risks with movement towards manufacture, ensuring the design of a robust and scalable process.
Building networks with academic groups and equipment manufacturers will undoubtedly advance flow capabilities within any CDMO, including Almac. Academic collaborations are essential to securing a pipeline of competent, expertly trained flow chemists with a crucial understanding of industry requirements. It is critical for academic institutions and industry to engage with each other to tailor undergraduate chemistry courses to ensure that continuous flow is a core discipline (akin to that of biocatalysis, for example). To this end, Almac has built collaborations within the island of Ireland to support its manufacturing sites in Northern Ireland and in the Republic of Ireland at Queens University Belfast and University College Dublin.
The so-called CDMO ‘cultural change’ will be accelerated if students are already exposed to new technologies and novel strategies for synthesis, building a deep understanding of the core principles.
Collaborations with flow equipment manufacturers also facilitates rapid development of flow processes through tapping into their expertise and identifying suitable engineering partners to translate lab processes to manufacturing.
Almac’s platform technology
With the adoption of flow chemistry increasing at research-scale, industry expects that continuous flow offerings available from the innovator should be scalable at the selected CDMO site(s). Since flow chemistry is not ‘one thing’ and no one tool addresses all needs / chemical space, CDMOs are developing a toolbox approach with modular, flexible skids to meet their clients’ needs. Identifying processes where flow can be utilized as an enabling technology to add value for clients is a key strategy for Almac, based on in-house expertise and partnering with equipment experts like Chemtrix.
Specializing in key areas of flow chemistry is not only advantageous for hardware selection, but is also beneficial for expertise development and debottlenecking within a CDMO. With this in mind, Almac has, and will continue to, focus on flow platforms for high-pressure hydrogenation, cryogenic, high energy and photo-redox chemistries.
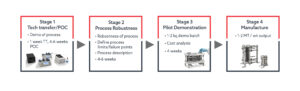
Almac’s flow chemistry department have implemented a four-stage project workflow to ensure successful delivery of projects for clients in acceptable timelines, with minimized risk and at competitive prices. These defined stages that ensure the development of robust, safe and scalable processes for multi-kilo to tonne-scale manufacture (Figure 3).
Future outlook
Flow chemistry at Almac and equipment manufacture at Chemtrix is not adventitious, offering clients the best available technology to meet the demanding processing needs of today whilst securing supply for the future. Continuous flow technology is being utilized at Almac to expedite the development of processes where the target chemistry is inherently difficult to scale in batch, with the synthesis of novel / highly valuable functional group interchanges using high energy and pressure, oxidation and photochemical transformations central to this.
Identifying opportunities to effectively implement the technology for appropriate chemical transformations is only part of the solution: access to modular, multi-purpose flow rig(s) is key to bringing about success as a CDMO. Through continued collaboration with academic and industrial partners, the technology will continue to grow within the pharmaceutical, specialities, agrochemical and flavour and fragrance (F&F) industries.
The time is now to change and flow into the future!
Authors:
Tom Moody1,2, Megan Smyth1, Scott Wharry1 and Charlotte Wiles3
1 Almac Sciences, 20 Seagoe Industrial Estate, Seagoe Road, Craigavon, BT63 5QD, Northern Ireland, UK www.almacgroup.com
2 Arran Chemical Company, Monklands Industrial Estate, Athlone, Co. Roscommon, Ireland www.arranchemical.ie
3 Chemtrix BV., Galvaniweg 8a, 6101 XH Echt, The Netherlands www.chemtrix.com
REFERENCES
- Baumann, M.; Moody, T. S.; Smyth, M.; Wharry, S.; Process Res. Dev., 2020, Article ASAP; DOI: 10.1021/acs.oprd.9b00524.
2(a). Gobert, S. R. L.; Kuhn, S.; Braeken, L.; Thomassen, L. C. J., Org. Process Res. Dev. 2017, 21, 531− 542. (b) Britton, J.; Raston, C. L., Chem. Soc. Rev. 2017, 46, 1250−1271. (c) Shukla, C. A.; Kulkarni, A. A., Beilstein J. Org. Chem. 2017, 13, 960−987. (d) Hartman, R. L.; McMullen, J. P.; Jensen, K. F., Angew. Chem., Int. Ed. 2011, 50 (33), 7502−7519.
- a). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/quality-considerations-continuous-manufacturing (b) L. Lee,T. F. O’Connor,X. Yang, C. N. Cruz, S. Chatterjee, R. D. Madurawe, C. M. V. Moore, L. X. Yu &J. Woodcock, J Pharm Innov. (2015) 10:191–199.
- Rivera, N. R.; Kassim, B.; Grogorov, P.; Wang, H.; Armenante, M; Bu, X.; Lekhal, A.; Variankaval, N.; Process Res. Dev., 2019, 23, 11, 2556-2561
- (a) Ley, S.V.; Fitzpatrick, D. E.; Ingham, R. J.; Myers, R. M.; Chem. Int. Ed., 2015, 54, 3449-3496; (b) Price, G. A.; Mallik, D., Organ, M. G.; J. Flow Chem., 2017, 7, 82-86.


